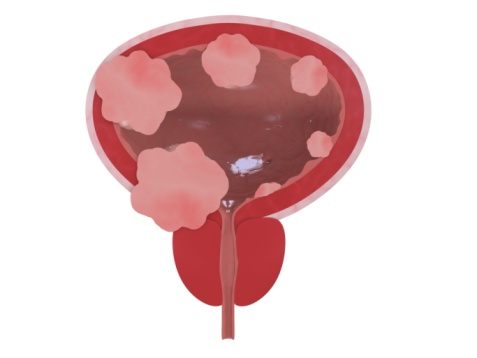
Thousands of bladder cancer diagnoses around the world have been linked to Takeda Pharmaceutical’s Type 2 diabetes drug Actos, prompting doctors to find better ways to treat the disease. A drug called Bacille Calmette-Guerin (BCG) is one of the newest treatments being used to combat it – and it’s good news for some bladder cancer victims. Treating Bladder Cancer Bladder cancer patients generally have limited treatment options —surgery to remove the tumor, and chemotherapy and/or radiation therapies. However, according to… Read More
Category: <span>Actos</span>

Takeda Pharmaceutical’s Type 2 diabetes drug Actos (pioglitazone) has been linked to bladder cancer in patients who were treated with the drug over the course of several years. However, the latest Actos bladder cancer victim says that he took Actos for only two years before being diagnosed with bladder cancer. He joins hundreds of others who are suing Takeda for failing to warn consumers about risks that many believe the company knew about years ago. Bladder Cancer Diagnosis Unthinkable The… Read More

The first of over 3,000 Actos bladder cancer lawsuits to go to trial resulted in a $6.5 million jury verdict against Japanese drug maker Takeda Pharmaceutics for failing to provide an adequate warning that its diabetes drug could cause bladder cancer. However, a judge later threw out that verdict on the basis of a doctor’s testimony. Although unfortunate, it is not the end of the road for Actos bladder cancer lawsuits. California Jury Said Takeda Should Have Warned Consumers A… Read More

Takeda Pharmaceuticals is currently defending the first of over 3,000 lawsuits filed against it alleging that its type 2 diabetes drug Actos caused or may have increased the risk of developing bladder cancer. Even though studies have conclusively shown this connection, the U.S. Food & Drug Administration (FDA) recently approved a generic version of Actos – leaving many patients, doctors and others in the medical community to ask the simple question – why? Studies Linking Actos To Bladder Cancer There… Read More

The trial in the first Actos bladder cancer lawsuit against Takeda Pharmaceutical Company is currently underway – and the result could determine how the Japan-based drug maker handles the 3,000 additional lawsuits pending in courts throughout the United States. The testimony presented thus far hasn’t been favorable for Takeda, as the plaintiff’s urologist recently told jurors that Actos is likely what caused his terminal bladder cancer. Actos Was “Most Substantial Factor” The plaintiff in the first Actos bladder cancer lawsuit… Read More
A recent Actos bladder cancer lawsuit alleges that Takeda Pharmaceutical Co., the Osaka Japan-based maker of Actos and Asia’s largest pharmaceutical company, used deceptive practices to keep the drug on the market for as long as possible to maintain sales. Court Documents Allege Takeda Secretly Surveyed Doctors Court documents filed in a recent Actos bladder cancer lawsuit allege that Takeda Pharmaceuticals became concerned that its diabetes drug could be linked to cancer and secretly surveyed doctors to see if they… Read More
The Driscoll Firm, LLC, is representing 38 plaintiffs named in the lawsuit filed in St. Clair County Circuit Court in Illinois, alleging that the manufacturer of Actos failed to warn of an increased risk of bladder cancer associated with the diabetes medication. St. Louis, MO (Vocus/PRWEB) February 23, 2013 – Nationally recognized drug injury lawyer John J. Driscoll announced today that his firm is representing 38 plaintiffs who have filed a lawsuit in St. Clair County Circuit Court in Illinois… Read More
The founder of The Driscoll Firm, LLC, says patients may have developed illness related to the diabetes drug prior to a June 2011 U.S. Food and Drug Administration (FDA) safety announcement and labeling change. St. Louis, MO (Vocus/PRWEB) January 25, 2013 – Nationally recognized drug injury lawyer John J. Driscoll said today that his firm is now reviewing potential injury claims arising from use of the Actos diabetes medication. According to the U.S. Food and Drug Administration (FDA), Actos (pioglitazone)… Read More
A personal injury lawsuit recently filed by a man who developed bladder cancer after taking Actos, a medication used to treat Type II diabetes, alleges that Actos manufacturer Takeda Pharmaceuticals withheld important safety information from the public about the drug’s increased risk of injury. Actos Injuries “Permanent & Severe” Those are the allegations contained in one of the most recent Actos bladder cancer lawsuits filed in the Circuit Court of Cook County by D. Steve Woody. Like many others, Woody… Read More
A new Scottish study finds that diabetes patients taking the group of diabetes drugs that includes Actos are at increased risk of suffering hip fractures. The University of Dundee researchers said bone fractures are “a severe adverse effect” for users of the class of diabetes drugs containing the active ingredients pioglitazone or rosiglitazone. The study of diabetes drug side effects appears in the November issue of Diabetologia, the journal of the European Association for the Study of Diabetes. The study… Read More