
GranuFlo / NaturaLyte Injury Lawyers
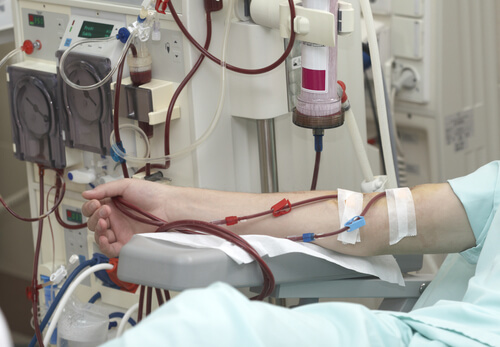
The Driscoll Firm, LLC, currently is investigating cases of metabolic alkalosis in kidney dialysis patients who were administered GranuFlo Dry Acid Concentrate or NaturaLyte Liquid Acid Concentrate. Metabolic alkalosis is a blood irregularity that can lead to heart arrhythmia, heart attack, convulsions, heart failure and/or coma.
The U.S. Food and Drug Administration (FDA) issued a Class I recall notice for these medical products in March 2012. Class I recalls are issued when there is a reasonable probability that use of the recalled products will cause serious adverse health consequences or death. They are the FDA’s highest level of recall.
If you or a loved one has suffered health problems after being administered GranuFlo or NaturaLyte products as part of their dialysis treatment, call The Driscoll Firm, LLC, today at our toll-free number or complete our online form. You can speak directly to an experienced defective medical products lawyer about your case.
Why Were GranuFlo and NaturaLyte Recalled?
GranuFlo Dry Acid Concentrate and NaturaLyte Liquid Acid Concentrate are given to dialysis patients to replace amino acids that are lost during hemodialysis.
The products are manufactured and distributed by Fresenius Medical Care North America, the largest dialysis services and products company in the world. Fresenius owns thousands of dialysis clinics. Fresenius also manufactures a large number of the dialysis machines and medical products used in dialysis care, including dialyzers, blood lines, needles and dialysis concentrates.
In issuing its recall of the GranuFlo and NaturaLyte products in March 2012, the FDA said that when the products are administered in improper doses, they can contribute to elevated bicarbonate levels in dialysis patients.
Elevated bicarbonate levels in a person’s body can cause a pH imbalance in the blood known as “metabolic alkalosis.” Metabolic alkalosis is considered a significant risk factor associated with:
- Low blood pressure
- Hypokalemia (low potassium levels)
- Hypoxemia (low oxygen levels)
- Hypercapnia (elevated carbon dioxide levels)
- Cardiac arrhythmia (irregular heart beat)
- Cardiopulmonary arrest (heart attack).
Metabolic alkalosis may lead to convulsions, heart failure and coma if it is not treated.
Investigations into Problems with GranuFlo
In May 2012, RenalWEB, a website devoted to dialysis, first reported the existence of a Fresenius Medical Care internal memo that had been issued in November 2011. The New York Times wrote an investigative piece about the same memo in June 2012.
According to these news sources, Fresenius alerted its own physicians and medical directors in November 2011 that GranuFlo “appeared to be contributing to a sharp increase in the risk of patients dying suddenly from cardiac arrest” at its clinics. But Fresenius did not warn other centers using the product until March 2012 – after the FDA had anonymously received a copy of the memo and began an inquiry, the New York Times said.
The New York Times, citing the Fresenius internal memo, reported that more than 900 dialysis patients had experienced sudden cardiac arrest in 2010 while undergoing hemodialysis at the hundreds of clinics operated by Fresenius.
Fresenius Medical Care treats more than a third of the estimated 400,000 Americans receiving dialysis, according to the New York Times. RenalWEB estimates that 125,000 patients in non-Fresenius clinics have been treated with GranuFlo.
Contact a GranuFlo / NaturaLyte Injury Lawyer Today
Lawsuits alleging injury and death among kidney dialysis patients who were administered GranuFlo or NaturaLyte are being filed across the country. If you or a loved one has used GranuFlo or NaturaLyte and suffered heart arrhythmia, heart attack, convulsions, heart failure, coma, death or other complications, do not delay.
Learn about your legal rights and options from an experienced GranuFlo / NaturaLyte injury lawyer today. Call The Driscoll Firm, LLC, at our toll-free number or use our online contact form. Compensation may include money for medical expenses, lost income, and your pain and suffering.
The personal injury and wrongful death lawyers at The Driscoll Firm, LLC, have a record of helping clients who have been harmed by dangerous pharmaceuticals and medical devices. Our founding attorney, John J. Driscoll, is a member of the Million Dollar Advocates Forum and the Multi-Million Dollar Advocates Forum.
For more information:
- Class I Recall, U.S. Food and Drug Administration
- “Dialysis Patient Survival Is Better at Fresenius Medical Care Clinics Because … — Part I,” RenalWEB
- “Dialysis Company’s Failure to Warn of Product Risk Draws Inquiry,” New York Times







